Abstract
Background: Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet count due to increased platelet destruction and suboptimal platelet production, and can lead to serious bleeding events. While some evidence on the epidemiology of ITP is available, such data are based on ex-US populations or are now over a decade old. Moreover, little data exist on the healthcare burden of ITP, especially in the early (i.e., 12-month) period following diagnosis. Accordingly, a new study was undertaken to characterize the incidence of ITP as well as healthcare utilization and expenditures during the early (e)ITP period in the US.
Methods: A retrospective cohort design and data from two healthcare claims repositories were employed; collectively, the repositories comprise claims information from ~50 million geographically diverse persons annually who are enrolled in over 75 US health plans. From the source population (i.e., enrolled persons from January 1, 2012 through December 31, 2015), patients with ITP were identified based on ≥1 hospitalization or ≥2 ambulatory encounters (separated by ≥30 days) with corresponding diagnosis codes (ICD-9: 287.31; ICD-10: D69.3); patients with causes of secondary thrombocytopenia and other selected conditions (e.g., viral hepatitis B, acute/chronic hepatitis C, HIV, lupus, pancytopenia, cancer) were excluded. ITP patients were stratified based on the year of initial diagnosis, and within each year-specific subset, those with ≥2 years of healthcare claims information available prior to their initial diagnosis, and no prior evidence of ITP during this period, were flagged as having newly diagnosed ITP. Annual incidence of ITP (per 100K persons) was weighted by age and sex to reflect the US population. Healthcare utilization and expenditures (2016US$) for eITP (i.e., during the first 12 months following diagnosis) were descriptively analyzed.
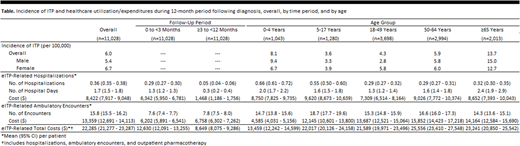
Results: Among the 198.1 million persons in the source population, 11,028 were newly diagnosed with ITP during the study period. Annual incidence of ITP in the US was estimated to be 6.0 per 100,000 persons, to be higher among women versus men (6.7 vs. 5.4 per 100,000), and to be highest among children aged 0-4 years (8.1 per 100,000) and adults aged ≥65 years (13.7 per 100,000) (Table). ITP patients averaged 0.36 hospitalizations and 15.8 ambulatory encounters during the 12-month period following diagnosis, and corresponding average expenditures were $8,422 and $13,359; including outpatient pharmacotherapy, total healthcare expenditures averaged $22,285. Hospitalizations were more common (0.29 vs. 0.05) and costly ($6,342 vs. $1,468) during the first three months following diagnosis versus the next nine months; numbers (7.6 vs. 7.8) and expenditures ($6,202 vs. $6,758) for ambulatory encounters were roughly comparable between periods. While hospitalizations were twice as common among children versus adults, mean hospital expenditures were similar, and expenditures for ambulatory encounters were substantially higher for adults versus young children.
Conclusion: The findings of this study suggest that nearly 20,000 persons are newly diagnosed with ITP each year in the US, and that ITP is much more common than previously reported. The findings also suggest that, among patients requiring formal medical care, the economic burden during the first 12 months following diagnosis is high, with US expenditures during this period totaling nearly $450 million.
Weycker:Amgen: Research Funding. Grossman:Amgen: Research Funding. Hanau:Amgen: Research Funding. Wu:Amgen: Research Funding. Hatfield:Amgen: Employment, Equity Ownership. Sharma:Amgen: Employment, Equity Ownership. Bensink:Amgen: Employment, Equity Ownership. Chandler:Amgen: Employment, Equity Ownership. Tarantino:Amgen: Membership on an entity's Board of Directors or advisory committees; Centers for Disease Control and Prevention: Research Funding; Health Resources and Services Administration: Research Funding; Pfizer: Other: Reviews grants; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Grifols: Research Funding, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal